

See the skin-clearing results from real patients with eczema who used once-daily, steroid-free VTAMA cream in clinical studies.
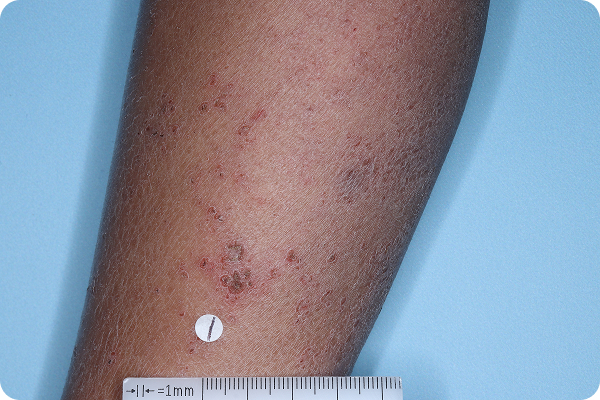
Age: 5
Gender: Male
Patient: 1028-021
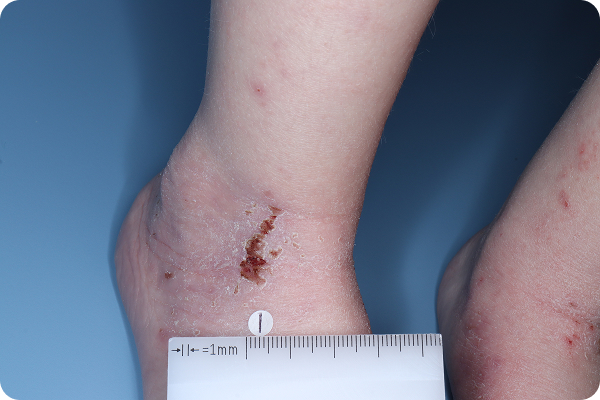
Age: 3
Gender: Male
Patient: 1028-007
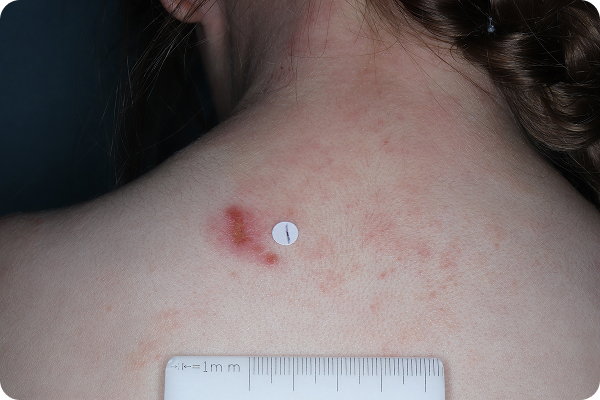
Age: 4
Gender: Female
Patient: 2005-007
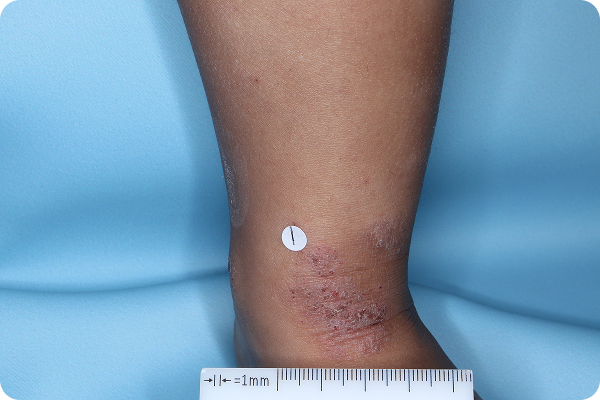
Age: 3
Gender: Male
Patient: 1032-009
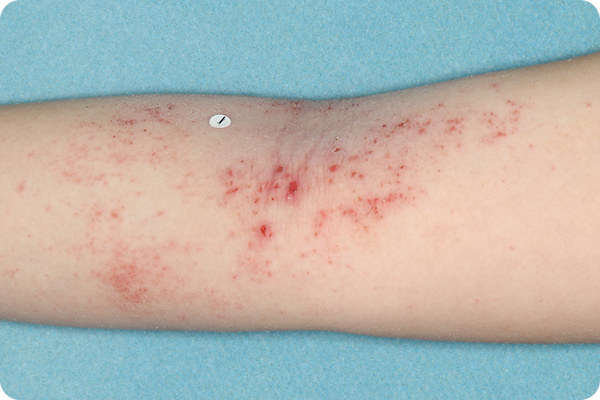
Age: 9
Gender: Female
Patient: 1020-002

Age: 54
Gender: Male
Patient: 1905-027
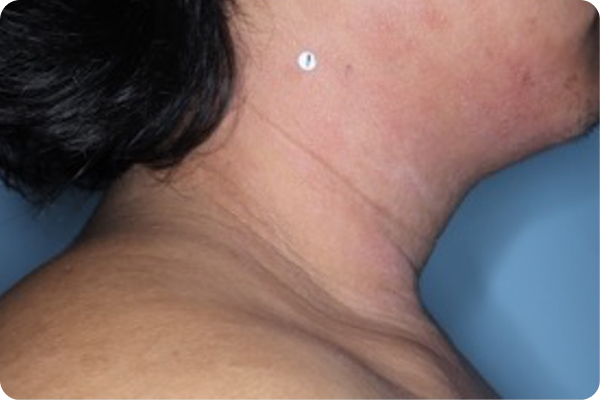
Age: 17
Gender: Male
Patient: 2005-016
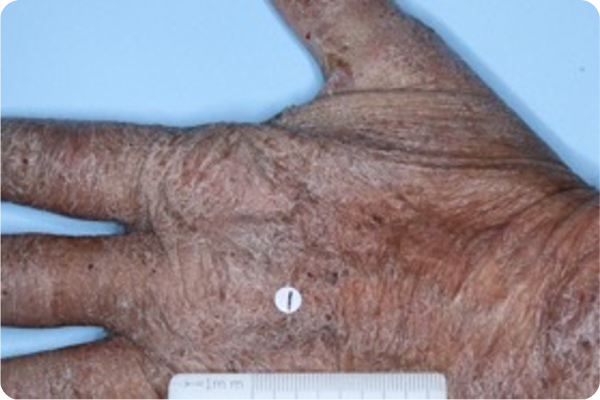
Age: 67
Gender: Male
Patient: 2005-001
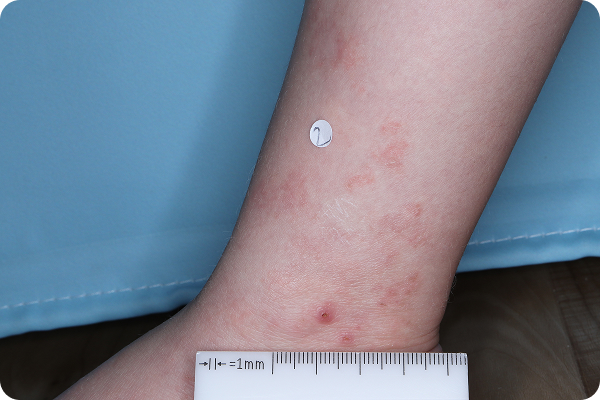
Age: 3
Gender: Female
Patient: 1032-007
Please note that the photographs you will see are representative of one area on individual patients treated with VTAMA cream during the pivotal clinical trials. Individual results may vary.
Please note that these photographs are representative of one area on a patient treated with VTAMA cream during the pivotal clinical trials. Individual results may vary.
Indications: VTAMA® (tapinarof) cream, 1% is an aryl hydrocarbon receptor agonist indicated for:
Adverse Events: In plaque psoriasis, the most common adverse reactions (incidence ≥1%) were: red raised bumps around the hair pores (folliculitis), pain or swelling in the nose and throat (nasopharyngitis), skin rash or irritation, including itching and redness, peeling, burning, or stinging (contact dermatitis), headache, itching (pruritus), and flu (influenza).
Adverse Events: In atopic dermatitis, the most common adverse reactions (incidence ≥1%) were: upper respiratory tract infection, red raised bumps around the hair pores (folliculitis), lower respiratory tract infection, headache, asthma, vomiting, ear infection, pain in extremity, and stomach-area (abdominal) pain.
You are encouraged to report negative side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088.
Please read the Prescribing and Patient Information for VTAMA cream and discuss it with your doctor.
 Back to top
Back to top
